SyMetric Clinical
Trial Platform
Built With
Purpose
Overview of Tools on Our Platform
Management
Management
Management
Identity and Access Management
Role-Based Access Control and Self-Service Account Management


Management
Organization Management
Organization Master

Management
Study Management
Study Master and Study Versioning


Management
Site Management
Site Master


Clinical Supplies Management
Inventory Management, Demand Forecasting, Automatic and Manual Shipment Requests, Site Distribution, Return Reconciliation, Retention Sample Management, and Temperature Tracking


Subject Management
Subject Screening, Enrolments, Visit Management, Randomization, Drug Dispensing, and Receipts


Management
Management
Coding
Services
Learning
Support
Management
Data Management
Global Library, Data Designer, Data Validation Manager, Data Collection, Discrepancy Management, Source Data Verification, and Data Exports


Management
Lab Management
Lab Master, Lab Ranges Manager, and Lab Data Management

Coding
Medical Coding
Auto Coder, Manual Coder and Supports WHO-DD & MedDRA Dictionaries


Services
Data Services
Third Party Integration Services and REST APIs
Learning
Digital Learning
Built-in Training Module, Online Tests, and Certifications





Support
Help and Support
Online Help Content and 24×7 Technical Support

Explore Our Solutions
IRT/IWRS
EDC
The Electronic Data Capture solution includes well-designed tools that transform the Data Management processes and simplify and automate the Data flow and validation of Data in Clinical Trials.
CTM
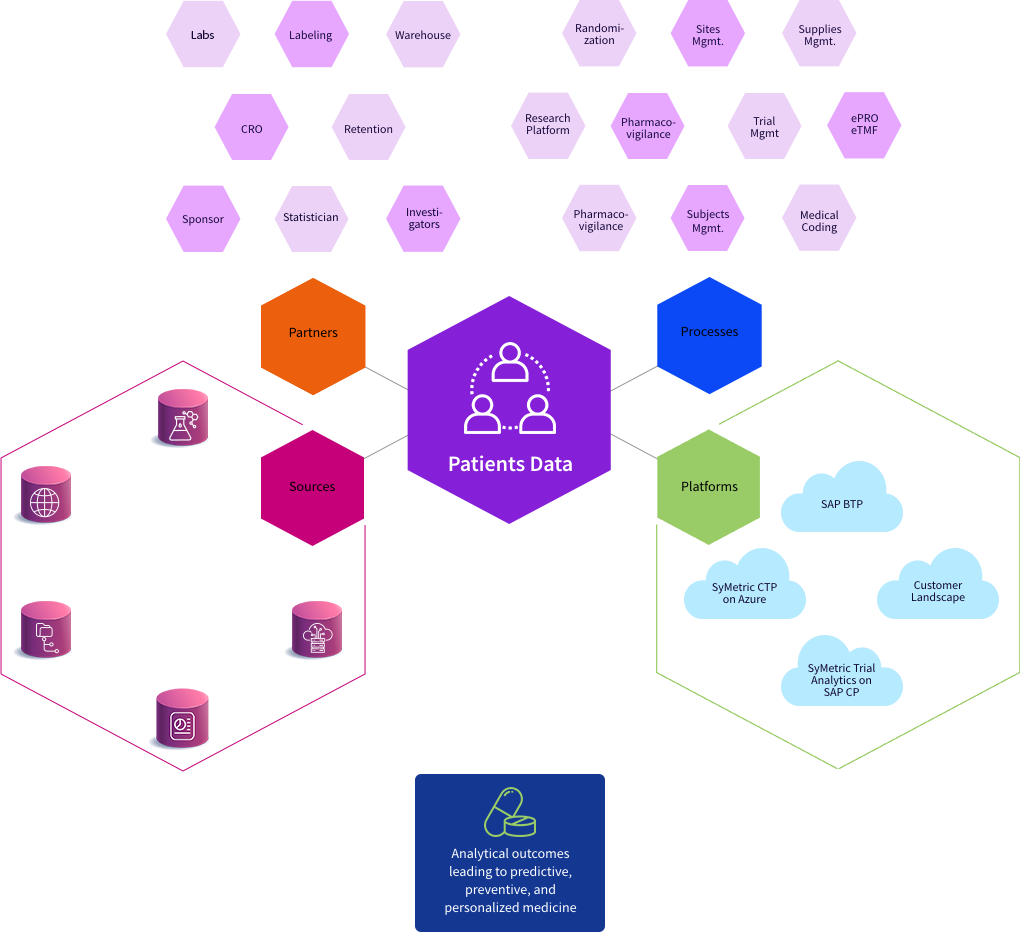
Our Collaboration Vision
Clinical Trials are conducted by numerous organizations which employ several highly skilled teams and individuals to perform unique and complex roles. For a Trial to be successful, the activities and tasks of these Teams and Individuals must be synchronized and documented.
The SyMetric Platform provides a virtual collaborative environment that is secure, user-friendly, and intuitive.
It streamlines the communication among the multiple teams involved in a Trial and provides the necessary tools for effective data capture and decision-making. This Collaboration Model along with fully customizable role-based authorizations enables teams and individuals with secure and real-time access to Trial Data.
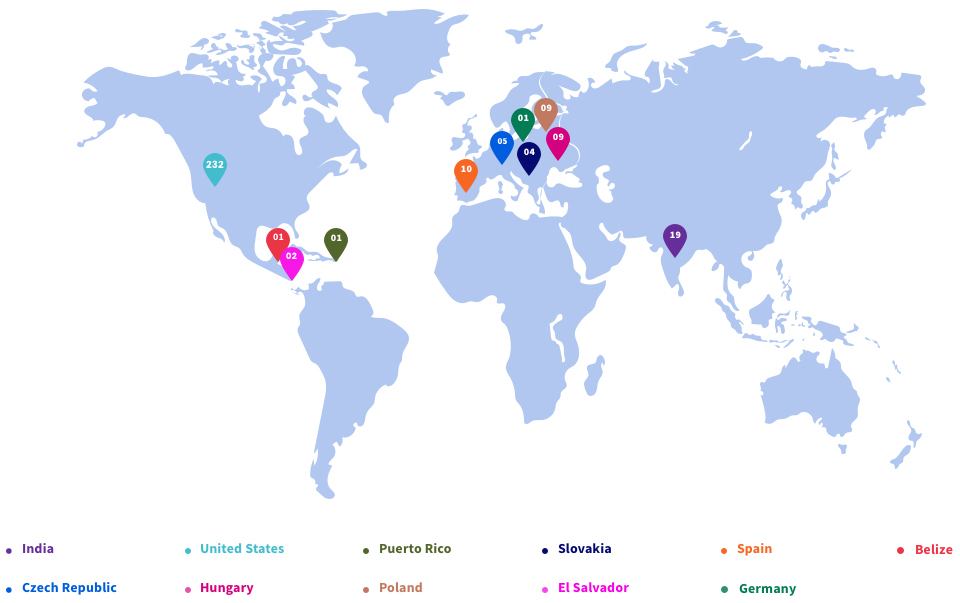
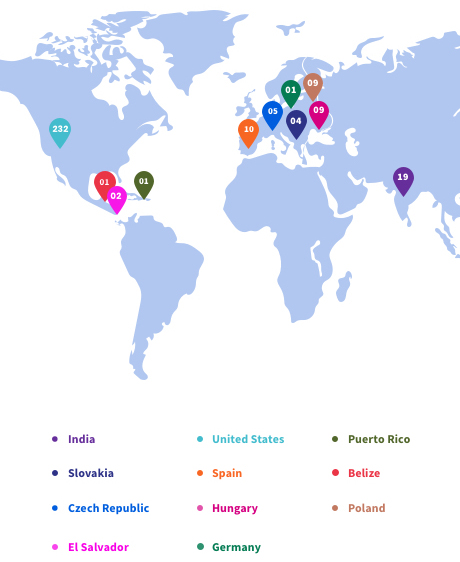
Affordable and Scalable, Globally

TITLE 01
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec
TITLE 02
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec
TITLE 03
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec
TITLE 04
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec
TITLE 05
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec
TITLE 06
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec
Looking for Customized Hosting
and Deployment Models?



The SyMetric Advantage
Fully Integrated Application with Modularized Experience
Increase trial efficiency and reduce costs with multicomponent (IWRS/IRT, CTM, and EDC) solutions built up as a single integrated solution.
Simple and Intuitive User Interface
A user-friendly layout complemented with a simple look and feel of the application assures enhanced user experience and increased control of the interface.
Single Sign-On Feature
The integrated cloud-based platform allows a Single Sign-On feature to streamline the user authentication process. The user can sign on to access all the authorized tools with a single set of credentials, avoiding cumbersome multiple sign-ins.
Highly Flexible and Customizable for Research studies
Tailored application design to suit all types of research study patterns with minimal configuration changes.
Reusable Forms and Business Rules
Import unique CRFs with standardized data definitions and associated business rules from the Global CRF Library to eliminate form re-designing efforts.
Compliant With 21 CFR Part 11 and ICH-GCP Guidelines
Our FDA 21 Part 11- and ICH-GCP-compliant application is featured with procedural controls, audit trails, and electronic signatures to safeguard the confidentiality and integrity of data.
Real-Time Reports and Dashboards
Visually track and analyze key performance indicators and site metrics for the study with customized reports and dynamic dashboard information.
24x7 Dedicated Technical Support
Expert technical team rigorously trained on the Application provides round-the-clock support for quick issue resolutions with minimal turnaround time.
Security and Compliance

ISO 9001
21 CFR Part 11 (US FDA)
ICH-GCP
Uncompromised Commitment to Data Privacy






